Phase change
Phase changes occur when Quantitative growth gives way to a qualitative leap. The various phase changes are shown below
graph LR; S[Solid] L[Liquid] G[Gas] S==>|Melting|L L==>|Freezing|S L==>|Vaporisation|G G<==>|Condensation|L S-->|Sublimation|G G-->|Deposition|S
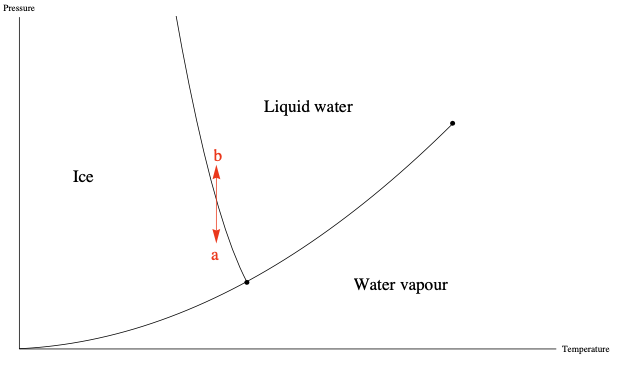
Phase changes occur at specific combinations of Temperature and Pressure, which can be shown in phase diagrams. The energy required to complete a phase change for a given mass, at a given temperature and pressure, is the Heat of transformation.
Diagrams